1
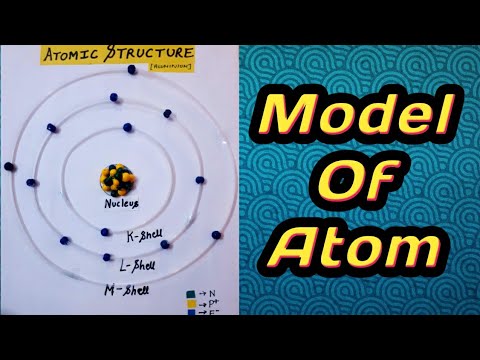
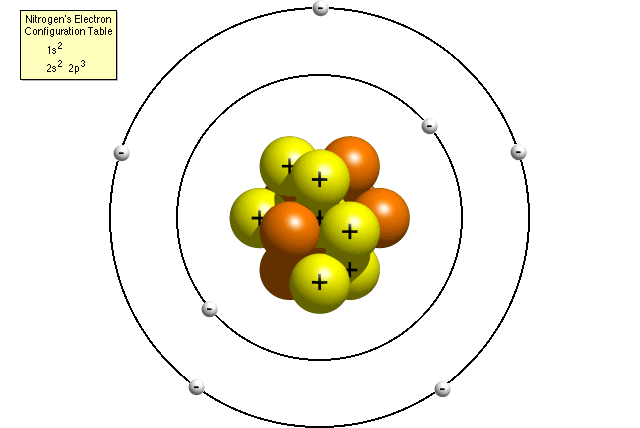
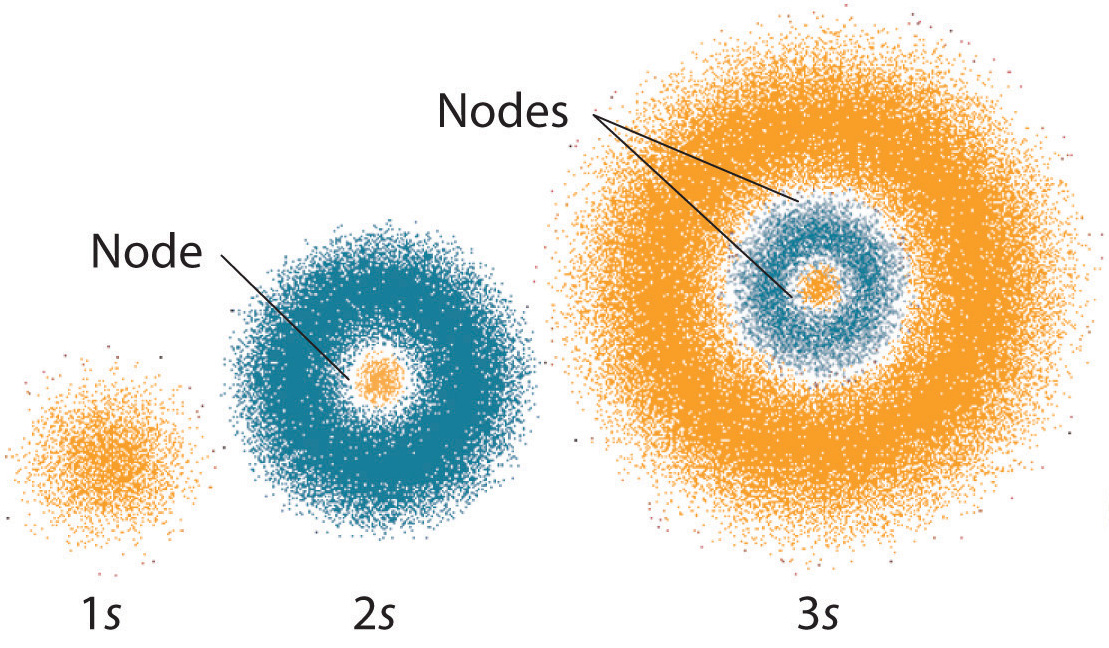
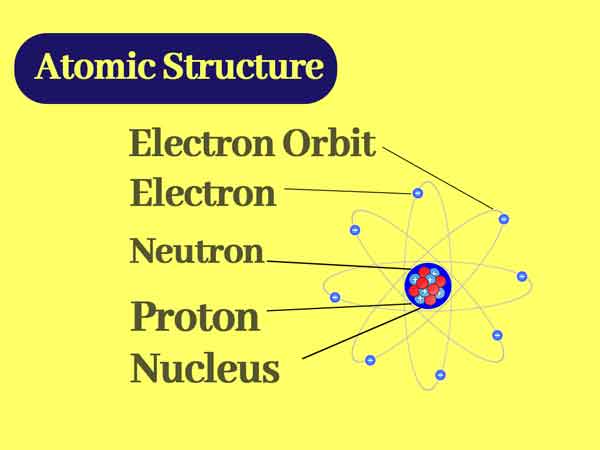
Challenge your students to build a model of an atom This atomic structure worksheet includes instructions, student planning sheet, and grading rubric so you have everything you need for a great science activity Students in third through fifth grade should be able to use their knowledge of atomic structure to complete this worksheet on atomsAtomic Structure Based on the Bohr Model Electrons occupy "shells" or orbits • These orbits are "quantized" • Identified by principle quantum number , n Energy of n th level for the hydrogen atom (and ONLY the H atom!) • Energy must be supplied to promote an electron from the ground state to an excited state
Rutherford atomic structure model
Rutherford atomic structure model-Answer There are various models of atomic structure At the beginning of around 1800's , the ancient Greek philosophers were considering atom as a indivisible particle which cannot be divided further But, eventually when the time passes, scientists startedLabel the information provided in the periodic table (use periodic table for ma Using a periodic table, complete the information in the chart below
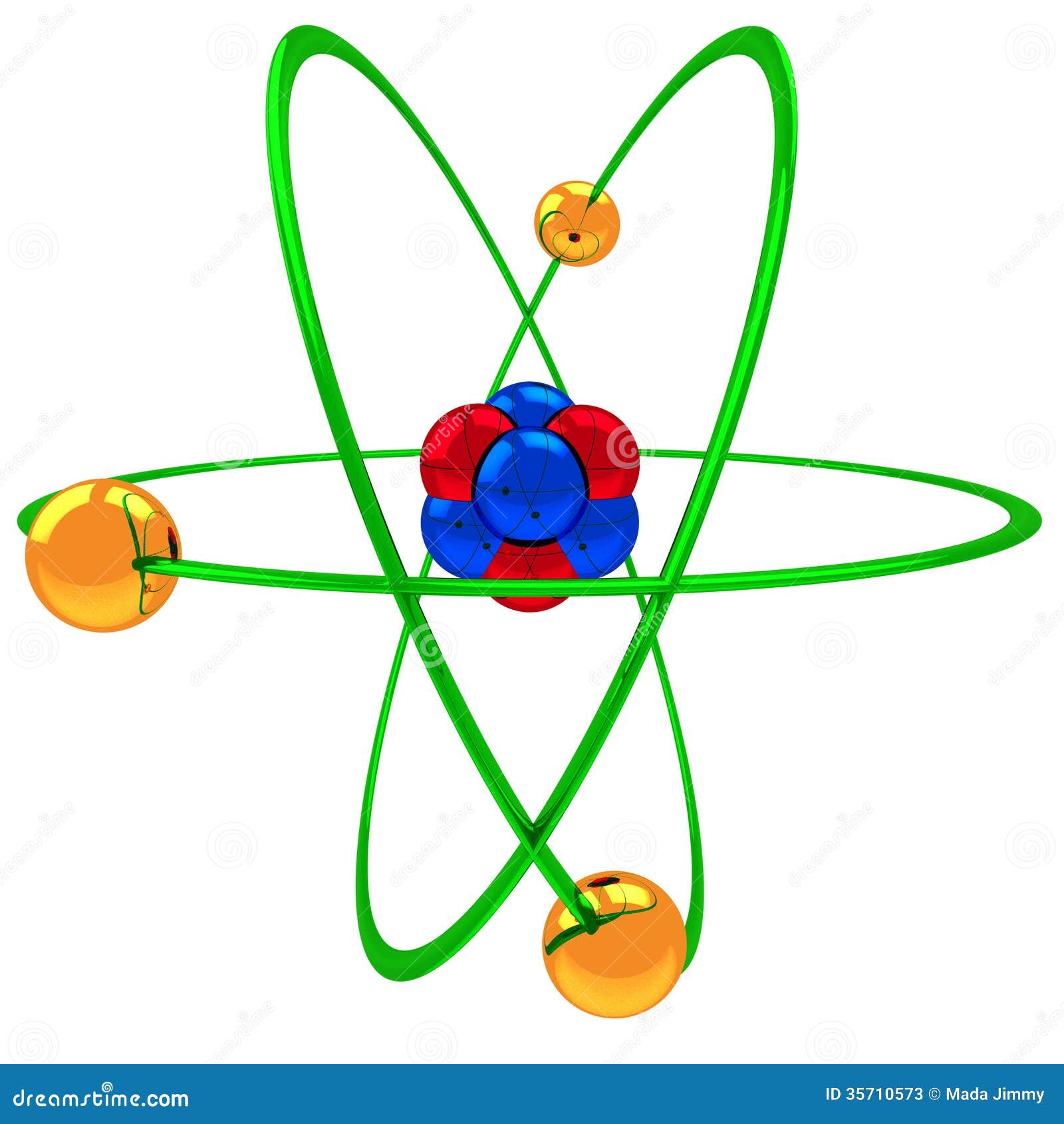
Atom Model Stock Illustration Illustration Of Atom Neutron
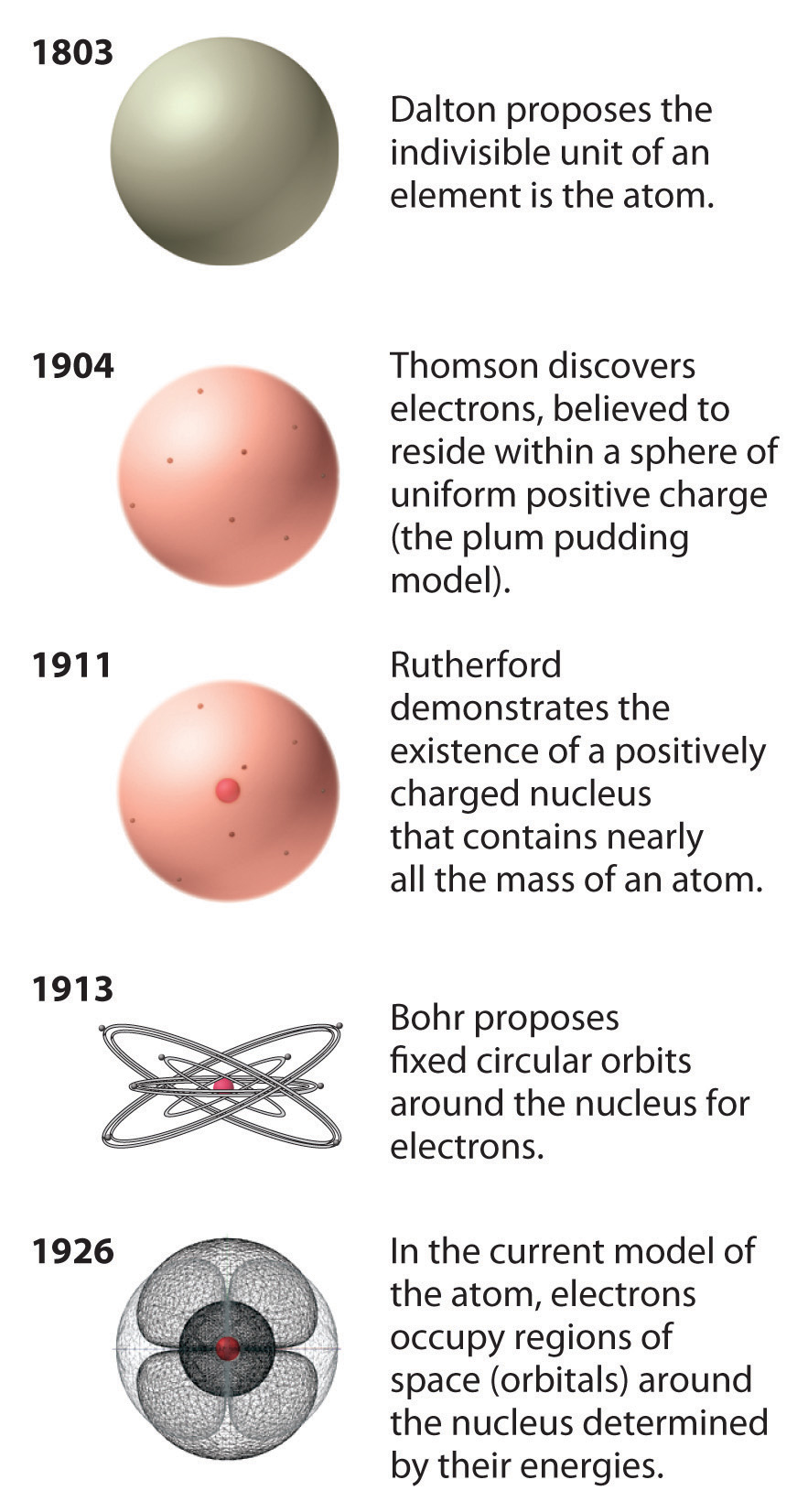
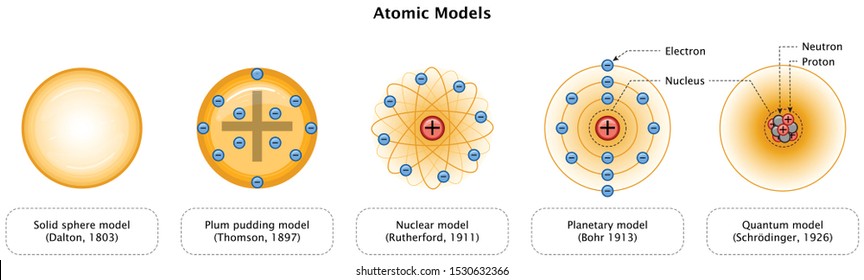
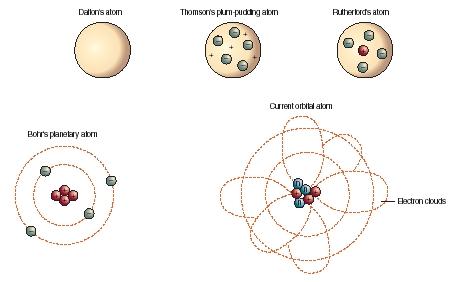
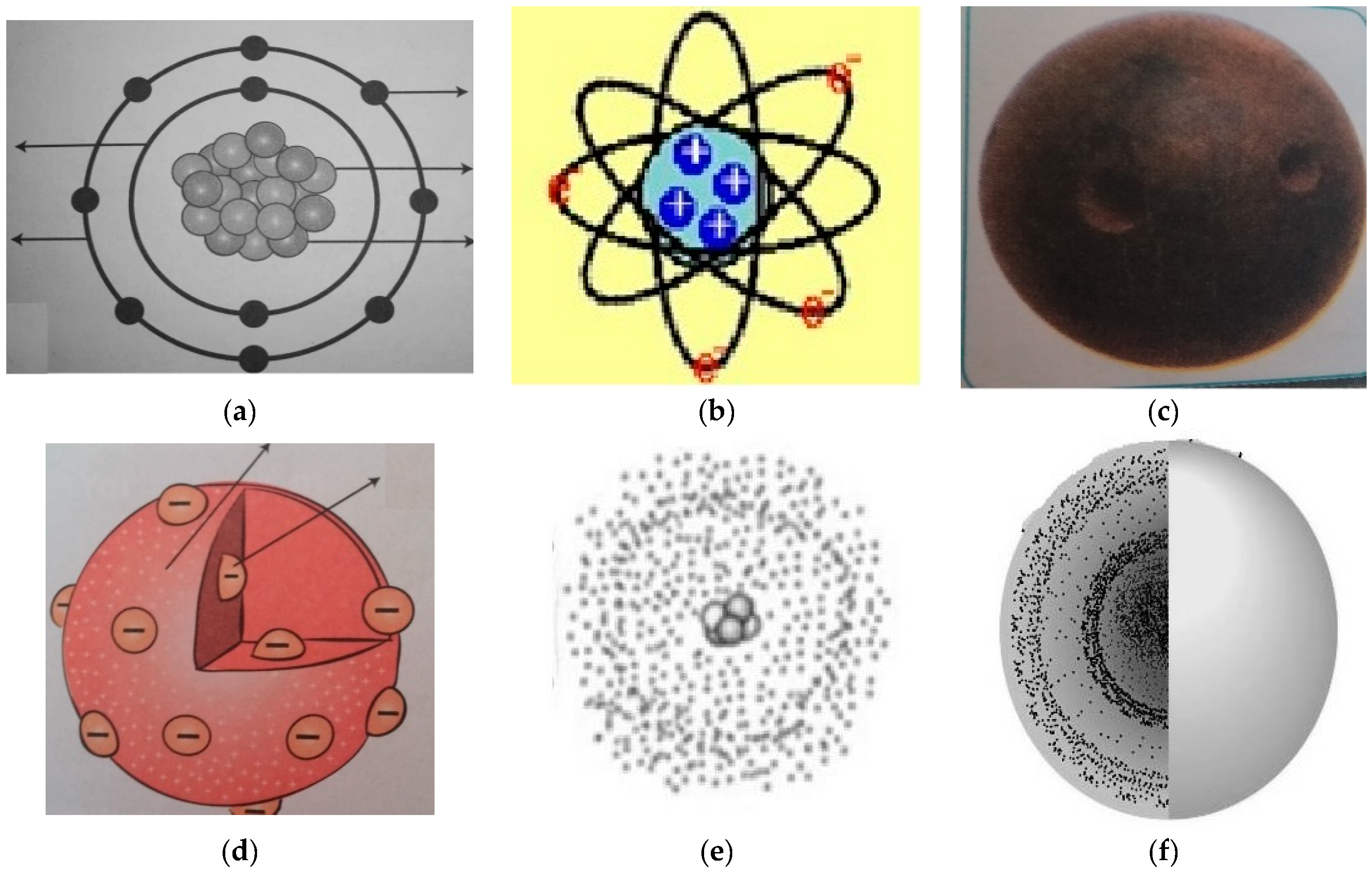
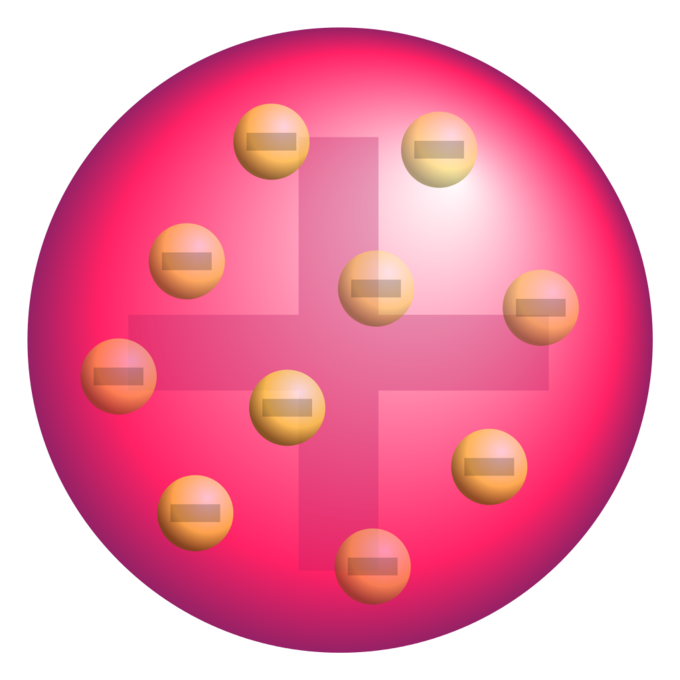
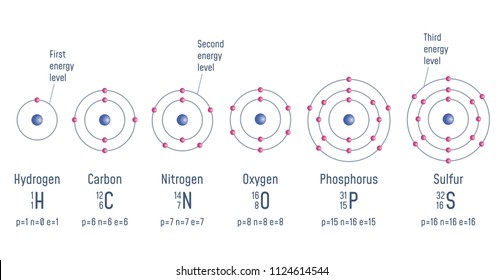
Various atomic models were proposed to explain the structure of the atom The aspirant will know about Thomson plumpudding model, Rutherford atomic model and Bohr's model of an atom Bohr's model was able to explain the spectra of the hydrogen atom talking about various spectral series like 1 Lyman Series spectrum Where Atomic model John Dalton Matter is made of small indivisible atoms Atoms can't be subdivided, created or destroyed 21 Atoms of theSince there were so many defects in Dalton's atomic theory, scientists started to carry out more experiments to explain the exact structure and the properties of an atom This led to the development of the modern atomic theory The modern atomic theory indicated the defects of Dalton's atomic theory
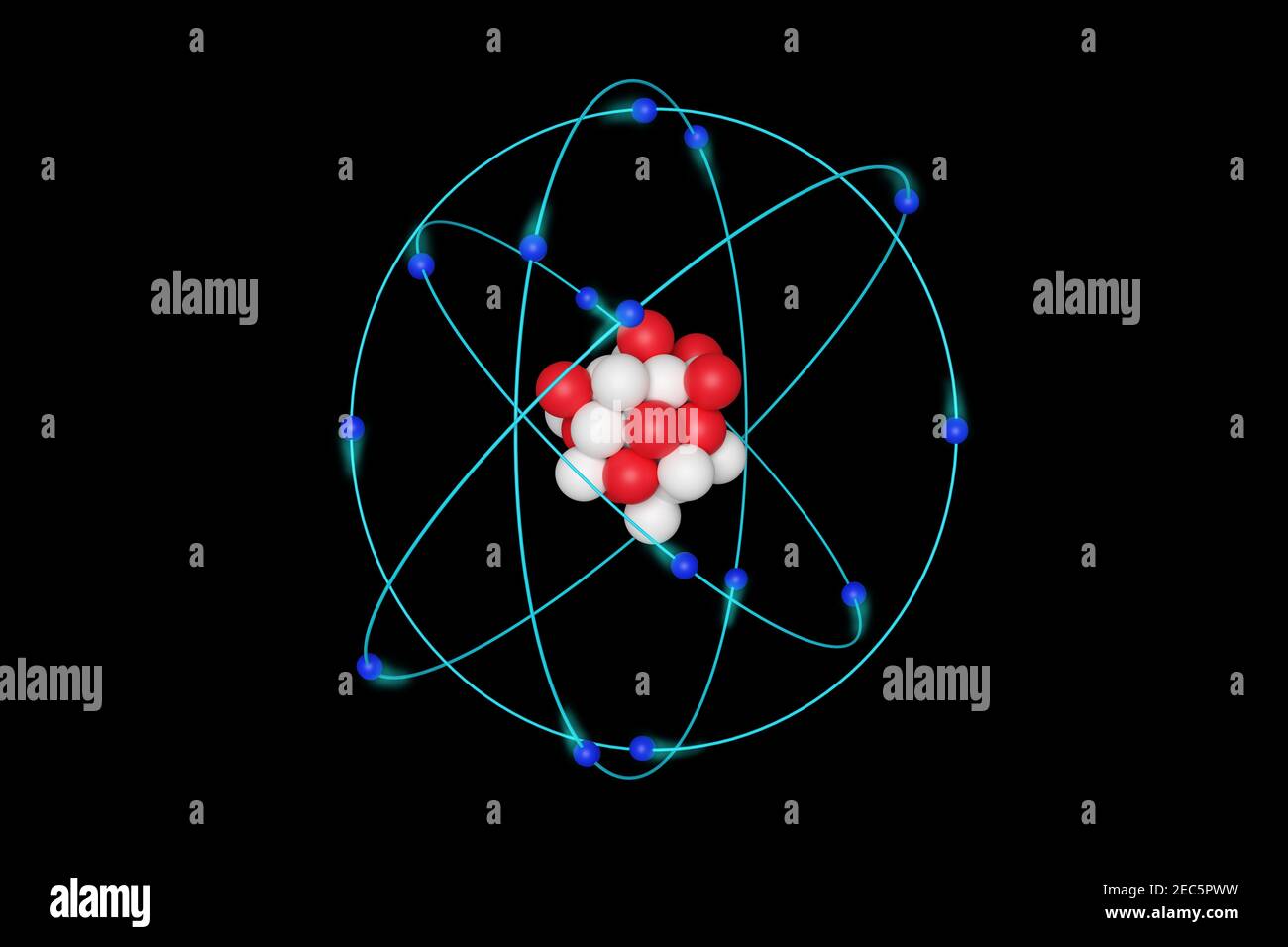
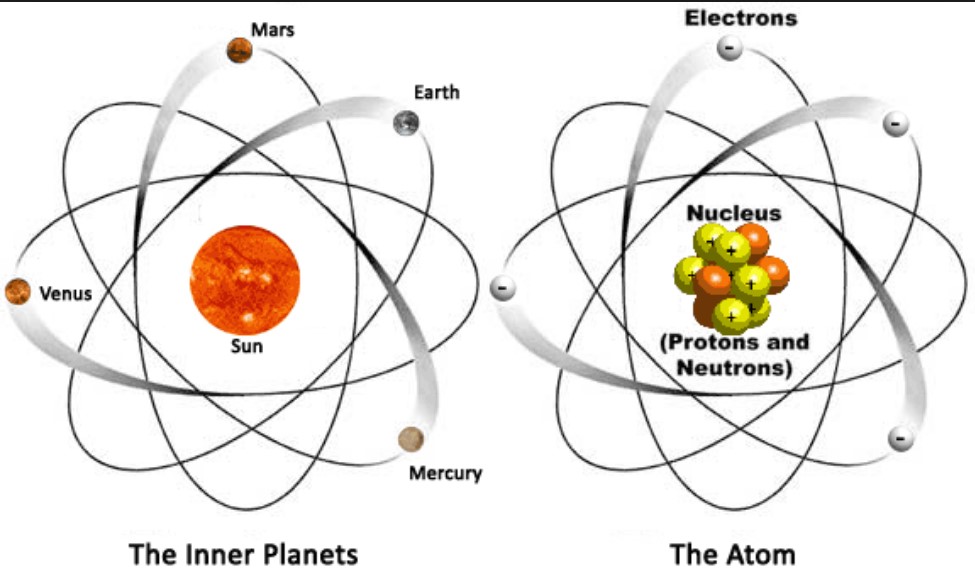
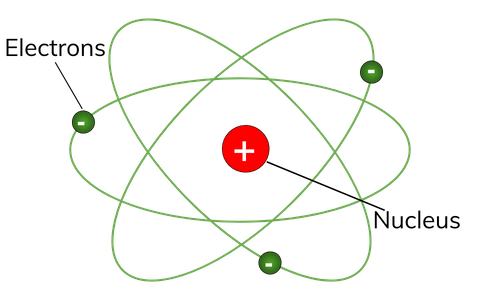
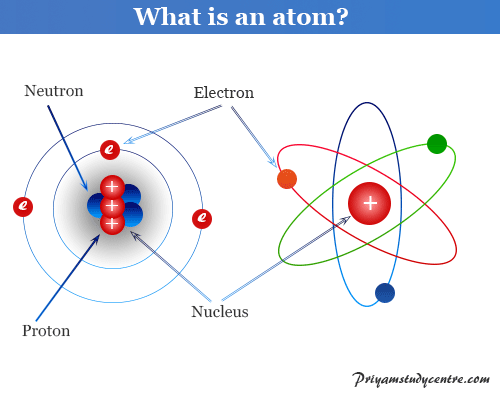
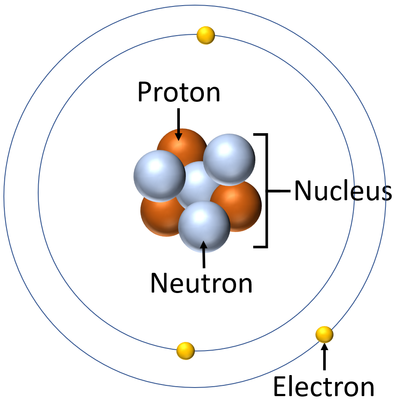
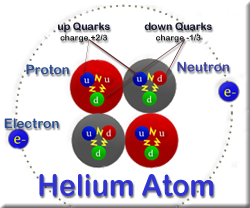
By the mid19s it had become apparent that the Bohr model was incorrect Scientists needed to pursue a totally new approach Two young physicists, Louis Victor de Broglie from France and Erwin Schrödinger from Austria, sug gested that because light seems to have both wave and particle characteristics (it behaves simultaneously as a wave and as a stream of particles), the electronDalton's experiments with gases led to some of the earliest measurements of atomic masses and a concept of atomic structure and reactivity Dalton's atomic theory contained the following ideas All atoms of a given element are identical The atoms of different elements vary in mass and size Atoms are indestructiblePostulates of Bohr atomic model Atom has a center known as a nucleus Electrons revolve around the nucleus in a fixed orbit with fixed energy and fixed velocity Electrons revolve only in those angular momentum which is integral multiple of nh/2 pi Here n is the shell number
Rutherford atomic structure modelのギャラリー
各画像をクリックすると、ダウンロードまたは拡大表示できます
 |  |  |
 |  |  |
 |  | |
「Rutherford atomic structure model」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 |  |  |
 | ||
「Rutherford atomic structure model」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 |  |  |
 |  |  |
「Rutherford atomic structure model」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 | ||
 |  |  |
「Rutherford atomic structure model」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 |  |  |
 |  |  |
「Rutherford atomic structure model」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 |  |  |
 |  | |
「Rutherford atomic structure model」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 |  | |
 |  |  |
「Rutherford atomic structure model」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 | ||
 | ||
「Rutherford atomic structure model」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 |  |  |
 |  |  |
「Rutherford atomic structure model」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 |  | |
 |  | |
「Rutherford atomic structure model」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  | |
 |  |  |
 |  |  |
「Rutherford atomic structure model」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 | 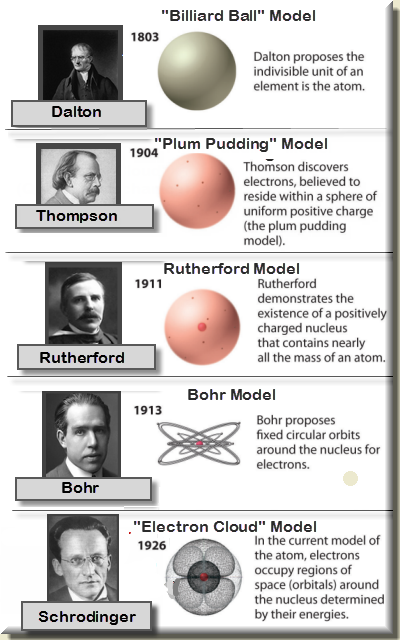 |
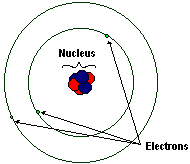
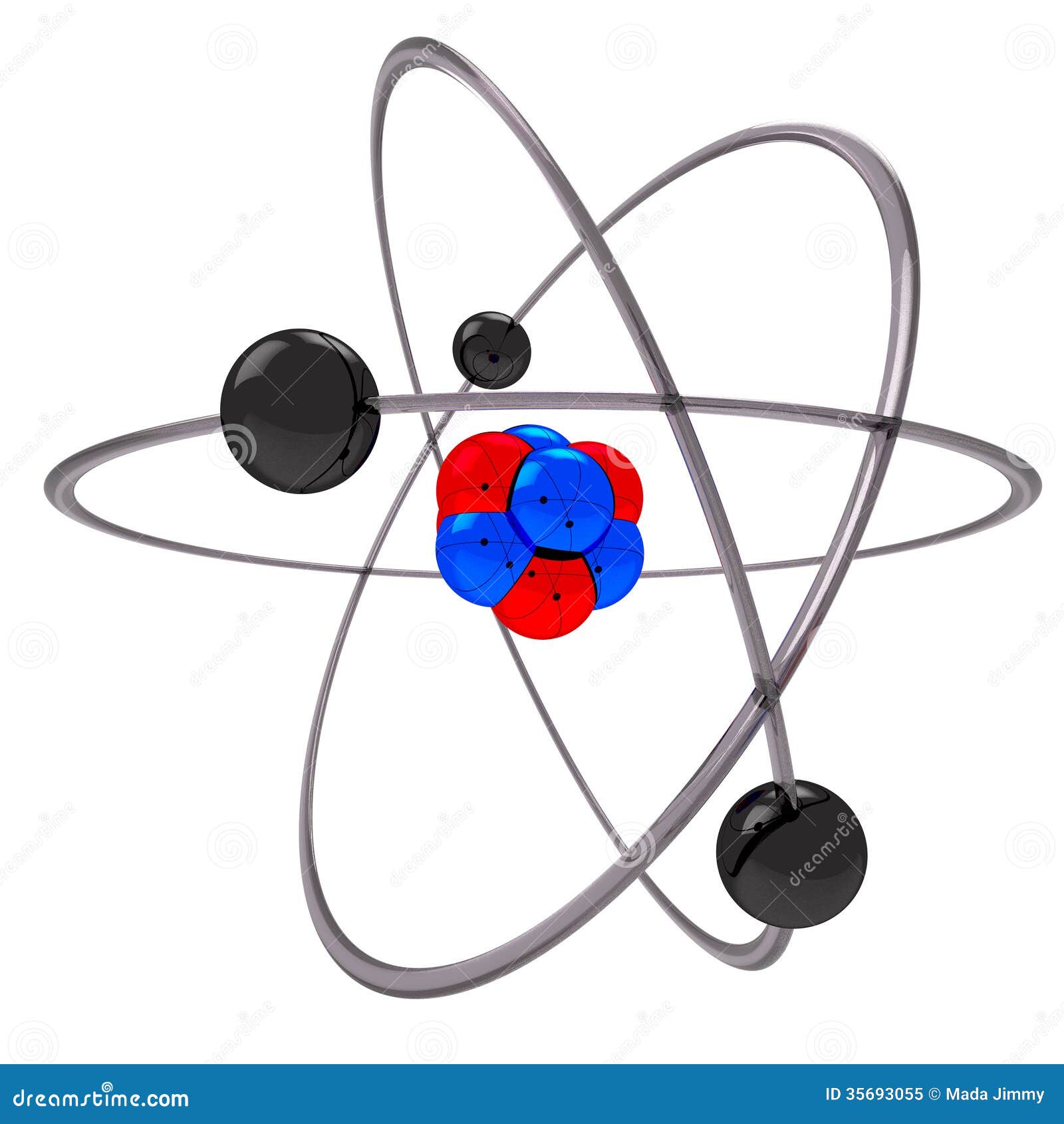
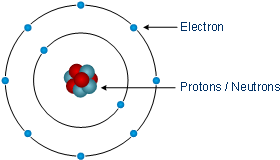
Rutherford model, description of the structure of atoms proposed (1911) by the New Zealandborn physicist Ernest Rutherford The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called electrons, circulate at some distance Rutherford model, description of the structure of atoms proposed (1911) by theThe early th century brought a succession of scientific models, or theories, to describe the atom and its components As experiments revealed more about subatomic particles, atomic models evolved from Thomson's "plum pudding model," to Rutherford's nuclear model, then to Niels Bohr's planetary model, and eventually to the currentlyaccepted quantummechanical model
Incoming Term: dalton atomic structure model, dalton's atomic structure model, alcl3 atomic structure model, rutherford atomic structure model, standard model of atomic structure, structured atomic model, modern model of atomic structure, model of atomic structure,




0 件のコメント:
コメントを投稿